-
04/18/25 Health Advisory: Stay alert for measles cases
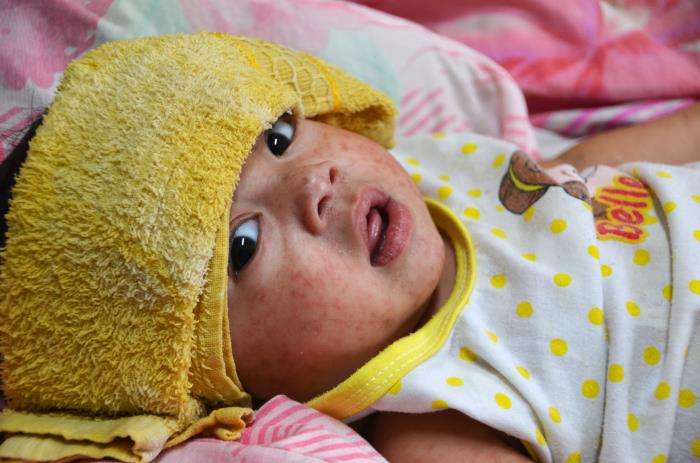
Summary: Recognize measles and notify the Health Department. Consider measles as a diagnosis in anyone who: If you suspect measles, call the Health Department before the patient leaves the clinic using . Background There is a large, ongoing measles outbreak in Texas. Several other states have reported smaller outbreaks of measles. Here in Washington, 4 cases have…
-
03/07/22 Health Advisory: Update on Johnson & Johnson vaccine ordering and guidance for healthcare settings
Advisory or Update, COVID-19, Health Advisory, Immunizations, News and Alerts, Notifiable Conditions, Provider ResourcesRequested actions Be aware, on March 4, Washington State Department of Health (DOH) discontinued ordering Johnson & Johnson COVID-19 vaccine because inventory is very low at Centers for Disease Control and Prevention (CDC) in Washington Immunization Information System (IIS). We encourage providers to post and look for available doses on IIS vaccine advertisement page. Email…
-
02/25/22 Health Advisory: COVID-19 Updates for Providers
Requested actions Be aware, on Feb. 22, Centers for Disease Control and Prevention (CDC) revised its COVID-19 vaccine clinical considerations. An 8-week interval between first and second doses of mRNA COVID-19 vaccine (Moderna and Pfizer) may be optimal for males 12–39 years old to reduce risk of myocarditis and increase efficacy. CDC still recommends a…
-
02/14/22 Health Advisory: CDC Updated Covid-19 Vaccination Guidelines; FDA Postponed its pediatric Covid-19 Vaccination Review
COVID-19 Updates for Providers Requested actions Be aware, on February 11, the Centers for Disease Control and Prevention updated their guidance for COVID-19 vaccination to include: For people who are moderately or severely immunocompromised: People who have completed a primary series of an mRNA vaccine (Pfizer-BioNTech or Moderna) are recommended to receive an mRNA booster…
-
02/01/22 Health Advisory: FDA Provides Full Approval For Spikevax
Advisory or Update, Communicable Disease and Immunization Update, COVID-19, Health Advisory, Notifiable Conditions, Provider Resources, UncategorizedCOVID-19 Updates for Providers Requested actions Be aware, U.S. Food and Drug Administration (FDA) granted full authorization to Moderna COVID-19 vaccine for people 18 years or older. Evidence shows the vaccine is safe and effective and manufactured with high quality. The vaccine will now be called Spikevax but has the same formulation as emergency use…
-
01/06/22 Health Advisory: Booster Eligibility Expanded
Advisory or Update, Communicable Disease and Immunization Update, COVID-19, Health Advisory, Immunizations, News and Alerts, Notifiable Conditions, Provider ResourcesCOVID-19 Updates for Providers Requested actions Be aware, Centers for Disease Control and Prevention (CDC) endorsed Advisory Committee on Immunization Practices’ (ACIP’s) decision to strongly recommend a single Pfizer COVID-19 vaccine booster dose for people 12–17 years old at least 5 months after their primary series. This expands the age of booster access and strengthens…
-
01/04/22 Health Advisory: CDC updated antiviral guidance
Advisory or Update, COVID-19, Health Advisory, News and Alerts, Notifiable Conditions, Provider ResourcesCOVID-19 Updates for Providers Requested actions Be aware, Centers for Disease Control and Prevention (CDC) updated its COVID-19 monoclonal antibody treatment recommendations. Sotrovimab monoclonal antibody formulation retains significant efficacy against Omicron, the dominant circulating variant. Other monoclonal antibody formulations under emergency use authorization (EUA)—bamlanivimab and etesevimab and casirivimab and imdevimab—lack clinical benefit against Omicron and…
-
12/22/21 Health Advisory: Paxlovid granted EUA and Testing before gathering
Advisory or Update, Communicable Disease and Immunization Update, COVID-19, Emerging Diseases and Conditions, Health Advisory, Immunizations, Infection Control, Notifiable Conditions, Provider Resources, Schools, Travel, Uncategorized, VaccineCOVID-19 Updates for Providers Requested actions Be aware, Food and Drug Administration (FDA) granted emergency use authorization (EUA) to the first oral antiviral treatment for COVID-19, Pfizer’s Paxlovid. The EUA comes after research showed Paxlovid was nearly 90% effective at preventing hospitalization and death in people 12 years or older at high risk of severe…
-
12/13/21 Health Advisory: COVID-19 Updates for Providers
Advisory or Update, Communicable Disease and Immunization Update, Health Advisory, MERS-CoV, News and AlertsCOVID-19 Updates for Providers Requested actions Be aware, Centers for Disease Control and Prevention (CDC) and Western States Scientific Safety Review Workgroup recommend everyone 16 years or older get a COVID-19 vaccine booster dose at the recommended interval. This recommendation comes after Food and Drug Administration (FDA) expanded its emergency use authorization (EUA) for Pfizer-BioNTech…
-
12/03/21 Health Advisory: COVID-19 Updates for Providers
Advisory or Update, COVID-19, Emerging Diseases and Conditions, Health Advisory, Infection Control, News and Alerts, Notifiable Conditions, Provider Resources, Schools, Travel, UncategorizedCOVID-19 Updates for Providers Requested actions Be aware, Centers for Disease Control and Prevention (CDC) issued a health advisory warning of COVID-19 variant of concern Omicron (B.1.1.529). At least one case has been identified in the U.S.; it was linked to travel. Omicron has not been detected in Pierce County; however, we are tracking its…
-
11/23/21 Health Advisory: COVID-19 vaccine booster doses available to everyone 18 years or older
Communicable Disease and Immunization Update, COVID-19, Immunizations, News and Alerts, Provider ResourcesCOVID-19 Updates for Providers Requested actions Be aware, following the Food and Drug Administration’s (FDA’s) decision to update its emergency use authorizations (EUAs) to allow COVID-19 vaccine booster doses for everyone 18 years or older, Centers for Disease Control and Prevention (CDC) and Western States Scientific Safety Review Workgroup recommend: People 50 years or older…
-
Health Advisory: COVID-19 Cases Increasing in Multiple Countries
Emerging Diseases and Conditions, Health Advisory, Infection Control, News and Alerts, Notifiable ConditionsRequested actions Be aware COVID-19 cases are increasing globally. Get a detailed travel history for patients with fever and acute respiratory illness. Ask patients with suspected COVID-19 infection to wear a surgical mask. Evaluate them in a private room with the door closed, ideally an airborne infection isolation room. All healthcare personnel entering the room…
-
Health Advisory: Novel Coronavirus (2019-nCoV) Respiratory Infection
There is 1 lab-confirmed case of 2019-nCoV in Washington, a traveler who returned to Snohomish County from Wuhan, China. Requested actions Get a detailed travel history when evaluating patients with fever and acute respiratory illness. Suspect 2019-nCoV in patients with: Clinical Features Epidemiologic Risk Fever1 or signs/symptoms of lower respiratory illness (e.g., cough or shortness of…